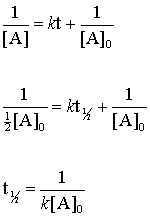half life formula for first order reaction
Plug in ½ for a use the time for x and multiply the left side by the initial quantity of the substance. Described as a function a quantity undergoing exponential growth is an exponential function of time that is the variable representing time is the exponent.
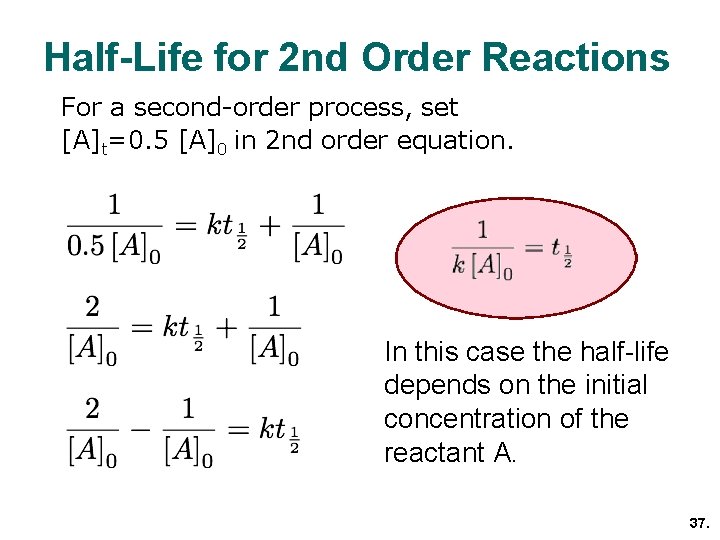
Chemical Kinetics Chapter 13 Topics 6 And 16
The gas constant R is also known as the universal molar or ideal gas constant.
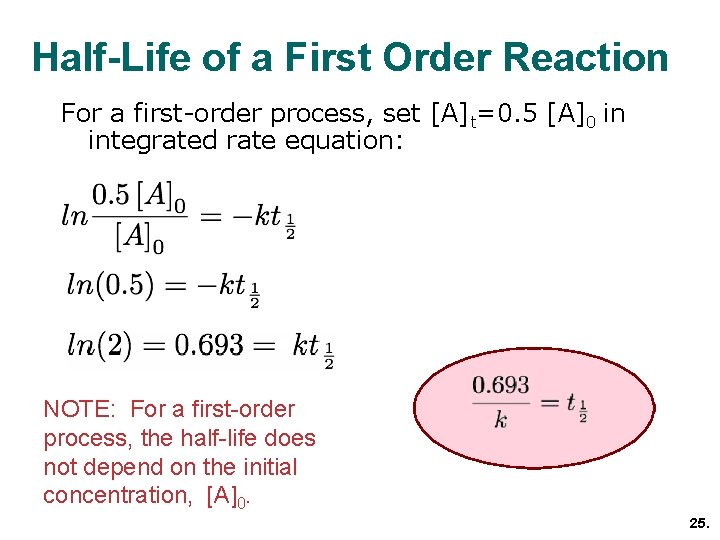
. It occurs when the instantaneous rate of change that is the derivative of a quantity with respect to time is proportional to the quantity itself. Michael Schumacher ˈ ʃ uː m ɑː k ər. Exponential growth is a process that increases quantity over time.
So in order to find the percent yield you need to calculate the theoretical yield. To find the half life of a substance or the time it takes for a substance to decrease by half youll be using a variation of the exponential decay formula. This gas constant referred to as a physical constant that is introduced in different fundamental equations in the physical sciences such as the ideal gas law the Arrhenius equation and the Nernst equation.
Born 3 January 1969 is a German former racing driver who competed in Formula One for Jordan Benetton Ferrari and MercedesSchumacher has a joint-record seven World Drivers Championship titles tied with Lewis Hamilton and at the time of his retirement from the sport in 2012 he held the records. You look at your chemical reaction and you see that your one and only limiting reactant is calcium carbonate. The Gas Constant R In PV nRT.

Integrated Rate Laws Chemistry For Majors

The Rate Law Concentration And Time Boundless Chemistry

First Order Reaction Definition Examples And Equations
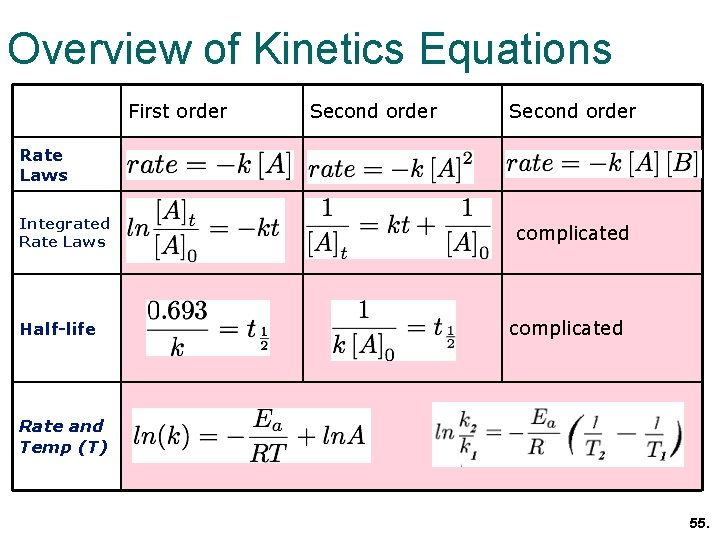
Chemical Kinetics Chapter 13 Topics 6 And 16
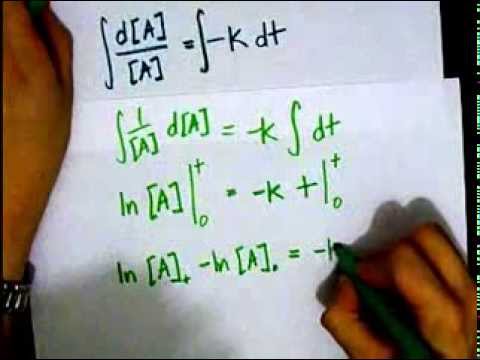
Integrated Rate Law First Order Reaction Youtube

Ppt Summary Of The Kinetics Of Zero Order First Order And Second Order Reactions Powerpoint Presentation Id 545041

Calculate The Half Life Of A First Order Reaction From Their Rate Constants Given Below A 200 S 1 B 2 Min 1 C 4 Year 1

Half Life Of A First Order Reaction Video Khan Academy

Integrated Rate Laws Zero First Second Order Reactions Chemical Kinetics Youtube

Chemical Kinetics Chapter 13 Topics 6 And 16

First Order Reactions Study Material For Iit Jee Askiitians

Half Life Introduction To Chemistry
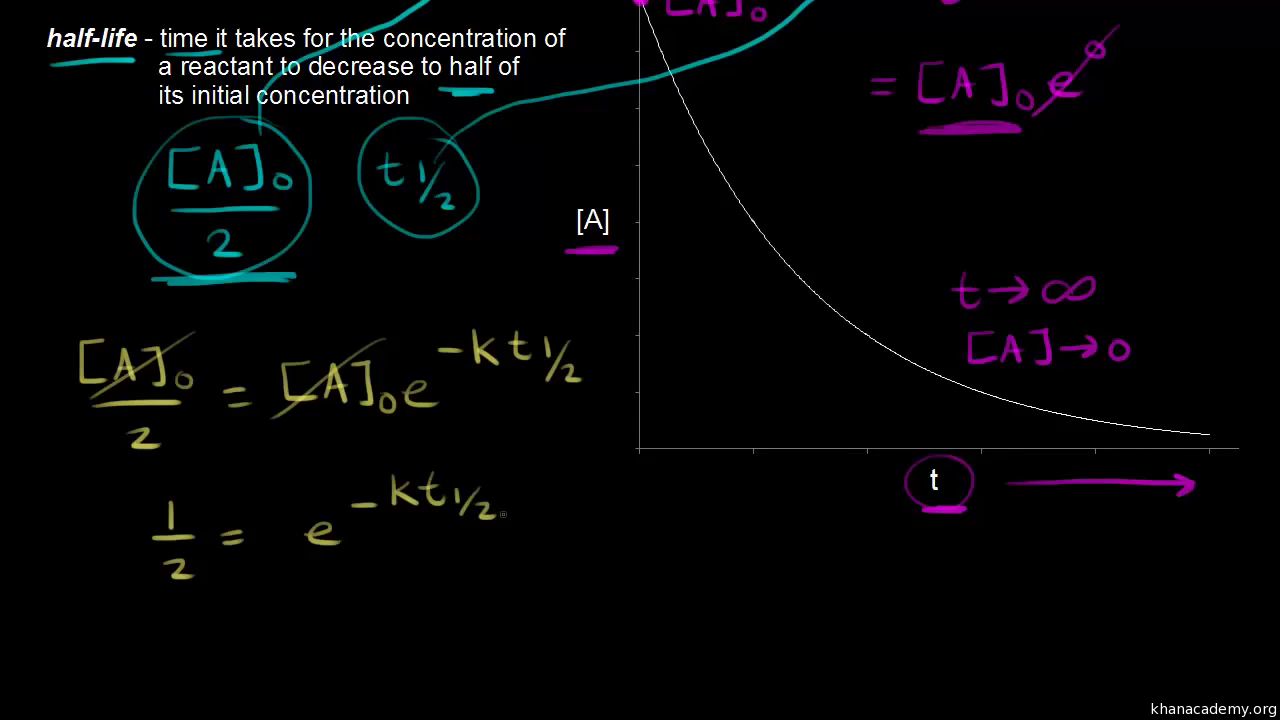
Half Life Of A First Order Reaction Video Khan Academy